Temperature
While temperature is related to thermal energy, there is no absolute correlation
between the amount of thermal energy (heat) of an object and its temperature.
Temperature measures the concentration of thermal energy in an object in much
the same way that density measures the concentration of matter in an object. As
a result, a large object will have a much lower temperature than a small object
with the same amount of thermal energy. As we shall see shortly, different
materials respond to changes in thermal energy with more or less dramatic
changes in temperature.
Video Lesson - What is Temperature
Degrees Celsius
In the United States, temperature is measured in degrees Fahrenheit (°F). However, Fahrenheit is not a metric
unit, so it will not show up on Physics. Physicists and non-Americans
usually talk about temperature in terms of degrees Celsius, a.k.a.
centigrade (°C). Water freezes at exactly
0°C and boils at
100°C. This is not a remarkable
coincidence—it is the way the Celsius scale is defined.
Physics won’t ask you to convert between Fahrenheit and Celsius, but if
you have a hard time thinking in terms of degrees Celsius, it may help to know
how to switch back and forth between the two. The freezing point of water is
0°C and 32°F.
A change in temperature of nine degrees Fahrenheit corresponds to a change of
five degrees Celsius, so that, for instance, 41°F
is equivalent to 5°C. In general, we can
relate any temperature of y°F to any
temperature of x°C with the
following equation:

Kelvins
In many situations we are only interested in changes of temperature, so it
doesn’t really matter where the freezing point of water is arbitrarily chosen to
be. But in other cases, as we shall see when we study gases, we will want to do
things like “double the temperature,” which is meaningless if the zero point of
the scale is arbitrary, as with the Celsius scale.
Video Lesson - Converting Kelvin to Celsius
The Kelvin scale (K) is a measure of absolute temperature, defined so
that temperatures expressed in Kelvins are always positive. Absolute zero,
0 K, which is equivalent to
-273°C, is the lowest theoretical
temperature a material can have. Other than the placement of the zero point, the
Kelvin and Celsius scales are the same, so water freezes at
273 K and boils at
373 K.
Definition of Temperature
The temperature of a material is a measure of the average kinetic energy of the
molecules that make up that material. Absolute zero is defined as the
temperature at which the molecules have zero kinetic energy, which is why it is
impossible for anything to be colder.
Solids are rigid because their molecules do not have enough kinetic energy to go
anywhere—they just vibrate in place. The molecules in a liquid have enough
energy to move around one another—which is why liquids flow—but not enough to
escape each other. In a gas, the molecules have so much kinetic energy that they
disperse and the gas expands to fill its container.
Heat
Heat is a measure of how much thermal energy is transmitted from one body to
another. We cannot say a body “has” a certain amount of heat any more than we
can say a body “has” a certain amount of work. While both work and heat can be
measured in terms of joules, they are not measures of energy but rather of
energy transfer. A hot water bottle has a certain amount of thermal energy; when
you cuddle up with a hot water bottle, it transmits a certain amount of heat to
your body.
Calories
Like work, heat can be measured in terms of joules, but it is frequently
measured in terms of calories (cal). Unlike joules, calories relate heat
to changes in temperature, making them a more convenient unit of measurement for
the kinds of thermal physics problems you will encounter on Physics. Be
forewarned, however, that a question on thermal physics on Physics may be
expressed either in terms of calories or joules.
A calorie is defined as the amount of heat needed to raise the temperature of
one gram of water by one degree Celsius. One calorie is equivalent to
4.19 J.
1cal = 1 g/°C = 4.19 J
You’re probably most familiar with the word calorie in the context of a
food’s nutritional content. However, food calories are not quite the same as
what we’re discussing here: they are actually Calories, with a capital “C,”
where 1 Calorie =
1000 calories. Also, these Calories are not
a measure of thermal energy, but rather a measure of the energy stored in the
chemical bonds of food.
Specific Heat
Though heat and temperature are not the same thing, there is a correlation
between the two, captured in a quantity called specific heat,
c. Specific heat measures how much
heat is required to raise the temperature of a certain mass of a given
substance. Specific heat is measured in units of J/kg ·
ºC or cal/g ·
ºC. Every substance has a different specific heat, but specific heat is a
constant for that substance.
Video Lesson - Specific Heat Capacity Equation
For instance, the specific heat of water,
Cwater,
is
4.19 × 103
J/kg · ºC or 1 cal/g ·°C. That means it takes
4.19 × 103
joules of heat to raise one kilogram of water by one degree Celsius. Substances
that are easily heated, like copper, have a low specific heat, while substances
that are difficult to heat, like rubber, have a high specific heat.
Specific heat allows us to express the relationship between heat and temperature
in a mathematical formula:
Q = mc?T
where Q is the heat transferred to a
material, m is the mass of the
material, c is the specific heat of
the material, and
?T
is the change in temperature.
Example
4190 J of heat are added to 0.5 kg of water with an initial temperature of
12°C. What is the temperature of the water after it has been heated?
By rearranging the equation above, we can solve for
?T :
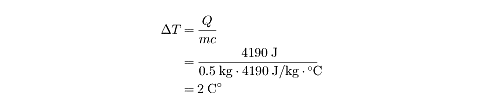
The temperature goes up by 2 C°, so if the initial temperature was 12°C, then
the final temperature is 14°C. Note that when we talk about an absolute
temperature, we write °C, but when we talk about a change in temperature, we
write C°.
Thermal Equilibrium
Put a hot mug of cocoa in your hand, and your hand will get warmer while the mug
gets cooler. You may have noticed that the reverse never happens: you can’t make
your hand colder and the mug hotter by putting your hand against the mug. What
you have noticed is a general truth about the world: heat flows spontaneously
from a hotter object to a colder object, but never from a colder object to a
hotter object. This is one way of stating the Second Law of Thermodynamics, to
which we will return later in this chapter.
Whenever two objects of different temperatures are placed in contact, heat will
flow from the hotter of the two objects to the colder until they both have the
same temperature. When they reach this state, we say they are in thermal
equilibrium.
Because energy is conserved, the heat that flows out of the hotter object will
be equal to the heat that flows into the colder object. With this in mind, it is
possible to calculate the temperature two objects will reach when they arrive at
thermal equilibrium.
Example
3 kg of gold at a temperature of 20°C is placed into contact with 1 kg of
copper at a temperature of 80°C. The specific heat of gold is 130 J/kg · °C
and the specific heat of copper is 390 J/kg · °C. At what temperature do the
two substances reach thermal equilibrium?
The heat gained by the gold,
Q = mcgold?Tgold
is equal to the heat lost by the copper,
Q = mccopper?Tcopper.
We can set the heat gained by the gold to be equal to the heat lost by the
copper, bearing in mind that the final temperature of the gold must equal the
final temperature of the copper:
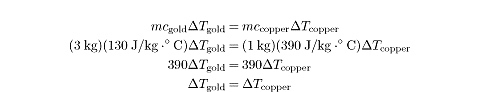
The equality between
?Tgold
and
?Tcopper
tells us that the temperature change of the gold is equal to the temperature
change of the copper. If the gold heats up by 30
Cº and the copper cools down by 30 C°, then
the two substances will reach thermal equilibrium at
50ºC.
Phase Changes
As you know, if you heat a block of ice, it won’t simply get warmer. It will
also melt and become liquid. If you heat it even further, it will boil and
become a gas. When a substance changes between being a solid, liquid, or gas, we
say it has undergone a phase change.
Melting Point and Boiling Point
If a solid is heated through its melting point, it will melt and turn to
liquid. Some substances—for example, dry ice (solid carbon dioxide)—cannot exist
as a liquid at certain pressures and will sublimate instead, turning
directly into gas. If a liquid is heated through its boiling point, it
will vaporize and turn to gas. If a liquid is cooled through its melting point,
it will freeze. If a gas is cooled through its boiling point, it will condense
into a liquid, or sometimes deposit into a solid, as in the case of
carbon dioxide. These phase changes are summarized in the figure below.
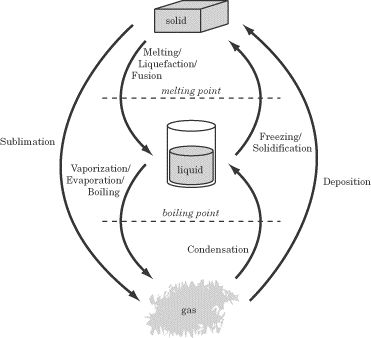
A substance requires a certain amount of heat to undergo a phase change. If you
were to apply steady heat to a block of ice, its temperature would rise steadily
until it reached 0ºC. Then the temperature would remain constant as the block of
ice slowly melted into water. Only when all the ice had become water would the
temperature continue to rise.
Latent Heat of Transformation
Just as specific heat tells us how much heat it takes to increase the
temperature of a substance, the latent heat of transformation,
q, tells us how much heat it takes
to change the phase of a substance. For instance, the latent heat of fusion
of water—that is, the latent heat gained or lost in transforming a solid into a
liquid or a liquid into a solid—is
3.3 × 105
J/kg. That means that you must add
3.3 × 105
J to change one kilogram of ice into water, and remove the same amount of heat
to change one kilogram of water into ice. Throughout this phase change, the
temperature will remain constant at 0°C.
The latent heat of vaporization, which tells us how much heat is gained
or lost in transforming a liquid into a gas or a gas into a liquid, is a
different value from the latent heat of fusion. For instance, the latent heat of
vaporization for water is
2.3 × 106
J/kg, meaning that you must add
2.3 × 106
J to change one kilogram of water into steam, or remove the same amount of heat
to change one kilogram of steam into water. Throughout this phase change, the
temperature will remain constant at 100°C.
To sublimate a solid directly into a gas, you need an amount of heat equal to
the sum of the latent heat of fusion and the latent heat of vaporization of that
substance.
Example
How much heat is needed to transform a 1 kg block of ice at –5°C to a puddle of
water at 10°C?
First, we need to know how much heat it takes to raise the temperature of the
ice to 0°C:
Q = mc?T = (1kg)(2.20 × 103J/kg.°C)(5°C) = 1.1 × 104J)
Next, we need to know how much heat it takes to melt the ice into water:
Q = mqfusion= (1kg)(3.3 × 105 J/kg) = 3.3 × 105 J
Last, we need to know how much heat it takes to warm the water up to
10ºC.
Now we just add the three figures together to get our answer:
1.1 × 104 + 3.3 × 105 + 4.2 × 104 = 3.8 × 106
Note that far more heat was needed to melt the ice into liquid than was needed
to increase the temperature.
Thermal Expansion
You may have noticed in everyday life that substances can often expand or
contract with a change in temperature even if they don’t change phase. If you
play a brass or metal woodwind instrument, you have probably noticed that this
size change creates difficulties when you’re trying to tune your instrument—the
length of the horn, and thus its pitch, varies with the room temperature.
Household thermometers also work according to this principle: mercury, a liquid
metal, expands when it is heated, and therefore takes up more space and rise in
a thermometer.
Any given substance will have a coefficient of linear expansion,
a,
and a coefficient of volume expansion,
ß.
We can use these coefficients to determine the change in a substance’s length,
L, or volume,
V, given a certain change in
temperature.
?L = aLi?T?V = ßVi?T
Example

A bimetallic strip of steel and brass of length 10 cm, initially at 15ºC, is
heated to 45°C. What is the difference in length between the two substances
after they have been heated? The coefficient of linear expansion for steel is
1.2
×
10–5/C°, and the coefficient of linear expansion for brass is 1.9
×
10–5/C°.
First, let’s see how much the steel expands:
?L = aLi?T= (1.2 ×l 10-5/C°)(0.1m)(30C°)= 3.6 ×10-5m
Next, let’s see how much the brass expands:
?L = aLi?T= (1.9 ×l 10-5/C°)(0.1m)(30C°)= 5.7 ×10-5m
The difference in length is
(5.7 ×10-5) - (3.6 ×10-5)m.
Because the brass expands more than the steel, the bimetallic strip will bend a
little to compensate for the extra length of the brass.
Thermostats work according to this principle: when the temperature reaches a
certain point, a bimetallic strip inside the thermostat will bend away from an
electric contact, interrupting the signal calling for more heat to be sent into
a room or building.
Methods of Heat Transfer
There are three different ways heat can be transferred from one substance to
another or from one place to another. This material is most likely to come up on
Physics as a question on what kind of heat transfer is involved in a
certain process. You need only have a qualitative understanding of the three
different kinds of heat transfer.
Video Lesson - Heat Transfer
Conduction
Conduction is the transfer of heat by inter-molecular collisions. For
example, when you boil water on a stove, you only heat the bottom of the pot.
The water molecules at the bottom transfer their kinetic energy to the molecules
above them through collisions, and this process continues until all of the water
is at thermal equilibrium. Conduction is the most common way of transferring
heat between two solids or liquids, or within a single solid or liquid.
Conduction is also a common way of transferring heat through gases.
Convection
While conduction involves molecules passing their kinetic energy to other
molecules, convection involves the molecules themselves moving from one
place to another. For example, a fan works by displacing hot air with cold air.
Convection usually takes place with gases traveling from one place to another.
Radiation
Molecules can also transform heat into electromagnetic waves, so that heat is
transferred not by molecules but by the waves themselves. A familiar example is
the microwave oven, which sends microwave radiation into the food, energizing
the molecules in the food without those molecules ever making contact with
other, hotter molecules. Radiation takes place when the source of heat is some
form of electromagnetic wave, such as a microwave or sunlight.
Next to display next topic in the chapter.
Practice Questions
Video Lessons and 10 Fully Explained Grand Tests
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.