Standard Prefixes
The metric system is fairly straightforward. The table of prefixes below is only
partial, but it includes the ones that you need to be familiar with for the
test.
|
Prefix |
Power |
Meaning |
Examples of measurements |
|
nano (n) |
10-9 |
one-billionth |
nanometer (nm): |
|
wavelength of light micro (m) |
10-6 |
one-millionth |
micrometer (mm): |
|
width of a hair milli (m) |
10-3 |
one-thousandth |
milliliter (mL): |
|
volume of acid in burette centi (c) |
10-2 |
one-hundredth |
centimeter (cm): |
|
length of paper deci (d) |
10-1 |
one-tenth |
deciliter (dL): |
|
amount of liquid kilo (k) |
103 |
one thousand times kilogram (kg): |
your weight |
Also useful to know are the units in the table below. Most of them will probably
be familiar to you.
|
What is being measured |
Common units |
|
length |
meter (m) |
|
mass |
gram (g) |
|
volume |
liter (L) or cm3 |
|
temperature |
degree Celsius (°C) and kelvin (K) |
|
time |
second (s) |
|
pressure |
kilopascal (kPa); atmosphere (atm); mmHg |
|
energy |
joules (?J);calorie (cal) kilopascal (kPa); |
|
amount of substance |
mole (mol) |
Scientific Notation
This is an easy way to express really large or really small numbers
conveniently. The general format for numbers expressed this way is
some number ×10some power
For instance, 6.022 × 1023
is really big, and 3.00 × 10-6
is really small. Notice that the proper position for the decimal is to the right
of the first nonzero digit. If you must move the decimal to get it into this
position, moving the decimal to the left makes the exponent appear larger, while
moving decimal to the right makes the exponent appear smaller. For example,
0.000567 in scientific notation would be 5.67 × 10–4.
You need to be able to handle numbers of this sort without a calculator.
Basically, you need to remember the following. For multiplication, add
exponents, and for division, subtract exponents. To get the log of
a value, raise it to the power of ten. This is mostly useful for pH
calculations. Now try some problems.
Example
(4.5 × 105)(3.0 × 108).
Explanation
The answer is 1.35 × 1014
(or rounded, 1.4 × 1014).
In solving this, think: 3 × 5
= 15, and then add the exponents: 5 + 8 = 13. Move the decimal to the right of
the first nonzero digit, or one place to the left.
Example
Try another one: 6.8 × 10
-2/0.2 × 10
10
Explanation
The answer is 3.4 × 10-12.
In solving this, think: 6.8/2 = 3.4, and then subtract the exponents: (-2) -
(10) = -12.
Example
Let’s try another: Find the log of 1.0×10-7.
Explanation
The answer is -7. The thought process is as follows. The log of 1.00 is 0. The
log of 10-7 is just the power of 10.
Temperature Conversions
The only two temperature scales that are needed for the SAT II Chemistry test
are the Celsius scale and the Kelvin scale. One degree on the
Celsius scale is the same increment as 1 kelvin on the Kelvin scale.
Celsius scale: This is the scale used in the chemistry laboratory for
most experiments. The freezing point of water is 0ºC, and the boiling point of
water is 100ºC. This was the original metric standard for temperature.
Kelvin scale: This is the scale used for working through gas law
problems. There are no negative numbers on this scale. At 0K, all motion
theoretically ceases.
Calculations Involving Metric Measurements (Dimensional Analysis)
Dimensional analysis offers an easy way to solve problems using
conversion factors and unit cancellations. Conversion factors are ratios
that equal 1. You know many of these ratios of equivalencies from everyday
living. For example, 1 gallon equals 4 quarts, 12 inches equals 1 foot, etc.
This is a useful technique for calculations that might come up on the test, so
work through the following problems to practice it.
Example
How many inches tall is a person who is 5 feet, 4 inches tall?
Explanation
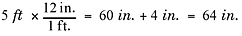
Example
How many milliliters would there be in 3.5 liters of soda?
Explanation
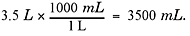
You’ll have to do plenty of conversions like the one above to solve problems on
the exam. Be sure that you are familiar with all the metric prefixes listed
earlier so that you can be successful when you need to convert numbers.
Density
Density is a complex unit. It is defined as mass per unit of volume:
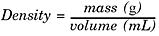
All pure substances have a unique density at a given temperature. Density is an
intensive physical property, meaning that it does not change with sample size.
Usually the solid form of a pure substance is denser than the liquid form of the
same substance. This makes sense because in most solids, the particles are much
closer together than in their liquid counterparts.
Typical units for density of solids and liquids are grams per milliliter or
grams per cubic centimeter. (Remember: 1 cm3 = 1 mL.) Typical units
for density of gases are grams per liter.
Example
Find the density of a substance that has a mass of 45.0 g and a volume of 3.0
mL.
Explanation
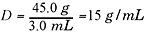
Example
What would be the mass of a substance that occupies a space of 2.0 cm3
and has a density of 7.5 g/cm3?
Explanation
D=M/V .Rearrange the equation to solve for mass: M = D × V.
Then
M = (7.5 g/cm3)(2.0 cm3) = 15 g
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.