Lesson: Chapter - 5
Intra- and Intermolecular Forces
Whether a particular group of bonded molecules takes the form of a solid, liquid,
or gas depends not only on the bonds that exist within each individual molecule,
but also on the presence and type of bonds between molecules. Hark back to the
different types of bonds we reviewed in the last chapter: ionic, covalent, and
metallic. Of these, ionic bonds tend to be the strongest, and this means that
substances that contain ionic bonds are solids at room temperature. Substances
that are primarily made up of covalent bonds, which are weaker, can be solid or
liquid, and their state will depend on the presence and type of
intermolecular forces.
The two main types of intermolecular forces that exist between molecules are
dipole-dipole forces (including hydrogen bonds) and London dispersion forces.
Dipole-Dipole Forces
Dipole-dipole attractions take place when two or more neutral, polar molecules
are oriented such that their positive (+) and negative (-) ends are close to each other.
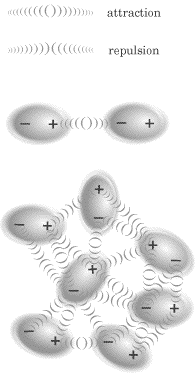
Because of the attraction between unlike charges, this is a fairly strong type of intermolecular force, and molecules held together by dipole-dipole forces tend to be in the solid or liquid state. Also, for molecules that are about the same size and weight, the strength of the dipole-dipole forces increases as the degree of polarity increases. In other words, the more polar a molecule is, the stronger the dipole-dipole forces it will form with itself and other molecules.
One very important and unique case of the dipole-dipole attraction is known as
hydrogen bonding. Hydrogen bonds are not true bonds: they’re just strong
attractive forces between the hydrogen on one molecule and a highly
electronegative atom on a nearby molecule.
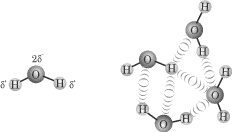
Hydrogen bonds most commonly form between hydrogen atoms and fluorine, oxygen, or
nitrogen. This type of intermolecular force is responsible for water’s unique
characteristics, such as its high specific heat and boiling point
temperature—but more about that later.
London Dispersion Forces—Weak Intermolecular Forces
London forces are relatively weak forces of attraction that exist between
nonpolar molecules and noble gas atoms, like argon (a noble gas) and octane (a
hydrocarbon; C8H18). These types of attractive forces are
caused by a phenomenon known as instantaneous dipole formation. In this
process, electron distribution in the individual molecules suddenly becomes
asymmetrical, and the newly formed dipoles are now attracted to one another.
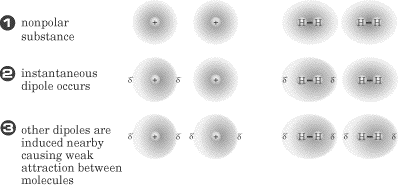
The ease with which the electron cloud of an atom can be distorted to become
asymmetrical is called the molecule’s polarizability. Think of this as a
probability issue. The greater the number of electrons an electron has, the
farther they will be from the nucleus, and the greater the chance for them to
shift positions within the molecule. This means that larger nonpolar molecules
tend to have stronger London dispersion forces. This is evident when you look at
the diatomic elements in group 7, the halogens. All of these diatomic elements
are nonpolar, covalently bonded molecules. Now, going down the group, fluorine
and chlorine are gases, bromine is a liquid, and iodine is a solid! For nonpolar
molecules, the farther you go down the group, the stronger the London dispersion
forces.
Next to display next topic in the chapter.
Practice Questions
Test Prep Lessons With Video Lessons and Explained MCQ
Large number of solved practice MCQ with explanations. Video Lessons and 10 Fully explained Grand/Full Tests.